Crystals have something magical about them when they appear to appear out of nowhere in a glass of water; they are in fact made up of substances already present in the liquid but in dissolved form. Perform a crystal experiment while learning the basics of the process.
Steps
Method 1 of 3: Making Simple Salt Crystals
Step 1. Heat some water in a saucepan
You just need a little, about 120 ml; heat it until the first bubbles begin to form.
- Children should ask for adult support to handle boiling water.
- Distilled water produces the best results, but tap water should be fine too.
- With heat, the water molecules accelerate.

Step 2. Choose the type of salt
There are different varieties and each leads to the formation of crystals with different shapes. Try the salts listed below and see what happens:
- Table salt needs a few days to turn into crystal; the "iodized" one doesn't work well, but you can still get some results;
- Epsom salt develops small crystals, similar to needles which, however, form in shorter times than those of table salt; you can buy it at the pharmacy;
- Alum develops quickly and often allows you to see some crystals after a few hours; you can buy it in supermarkets among the shelves dedicated to spices.
Step 3. Mix as much salt as possible
Remove the pan from the heat and pour about 50-100 g of salt into the water, stirring until the liquid becomes transparent again; if you don't notice any grains of salt in the water, add another spoonful. Keep pouring and stirring until the water is no longer able to dissolve the salt.
You just made one supersaturated solution; this means that the solvent (the liquid) contains more solute (salt) than it can usually hold under normal conditions.

Step 4. Pour the water into the clean jar
Proceed carefully and pour the supersaturated solution into a jar or other transparent and heat-resistant container; it should be as clean as possible so that nothing interferes with the development of the crystals.
- Slowly pour the liquid and stop just before the grains of salt fall into the jar. If you also transfer the fragments that are still solid, you run the risk that the crystals will grow around them instead of on the string.
- Since supersaturated solutions are very unstable, the salt will separate from the solution when you disturb it. This means that crystals will begin to form, which absorb the heat from the solution you have created.
Step 5. Add food coloring (optional)
A couple of drops of this substance change the color of the crystals; they may also cause smaller, lumpy crystals to develop, but they generally do not trigger large alterations.
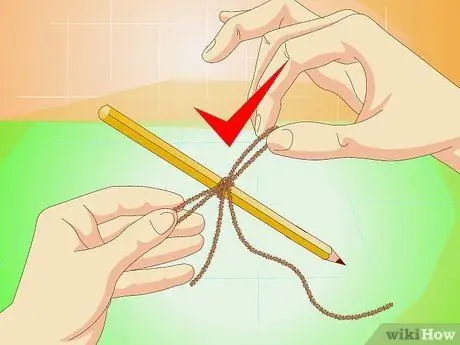
Step 6. Tie some string to a pencil
The latter should be long enough to rest transversely over the opening of the container; alternatively, you can use a popsicle stick or sprig.
The small cracks and rough edges of the string provide an anchor for the salt crystals and allow them to develop; do not use the fishing line, because it does not work as it is too smooth

Step 7. Cut the twine to the right length so that it hangs in the water
Only the submerged segment will become covered with crystals. Cut it short enough to avoid contact with the bottom of the jar, otherwise you will get small clusters of crystals.

Step 8. Rest the pencil balanced on the jar opening
The thread must hang and stretch out in the water; if the pencil does not stay still, fix it to the container with adhesive tape.
Make sure that the string does not touch the inside of the container, otherwise you will get small, lumpy crystals that adhere to the jar

Step 9. Move the container to a safe place
Put it out of the reach of animals and small children; here are some tips on this:
- To quickly get a cluster of crystals, expose the jar to the sun and / or hold it near a fan set at minimum speed; in this case, the crystals stop developing when they reach quite small size.
- If you prefer to have a single large crystal instead of a cluster, place the container in a cool, shady place; place it on a piece of polystyrene or another similar material that absorbs vibrations. There is a good chance that lumps will form anyway, but there should be single crystals inside.
- Epsom salt (and a few other less common salts) grows faster when refrigerated rather than exposed to the sun.

Step 10. Wait for the crystals to develop
Check the jar regularly to see if there are any small formations on the string; those of Epsom salt and alum grow quite quickly, within a few hours, but in some cases you have to wait a couple of days. Table salt usually takes a day or two to trigger development, but sometimes it has to wait up to a week. Once you notice small crystalline structures on the string, they start getting bigger and bigger within a couple of weeks.
When the water cools, it contains more salt than it normally would. This is a very unstable condition; consequently, if stimulated in this sense, the dissolved salt "leaves" the water to "cling" to the string. As the water evaporates, the salt remains in the solution which becomes even more unstable encouraging the formation of crystals
Method 2 of 3: Making a Single Large Crystal

Step 1. Make a handful of salt crystals
Follow the instructions described in the first part of the article, but using distilled water and without the support of the string or pencil. Simply leave the supersaturated solution in the vessel; within a few days a layer of crystals should form at the bottom.
- Use a large, flat, shallow container instead of the jar; in this way, it is easier for single, non-fused crystals to grow.
- Epsom salt is not very suitable for this method; give it a try with tableware or alum, or read the variations section for more ideas.

Step 2. Choose the crystal core
When they are ready, throw the liquid away and observe the crystals; lift them up with tweezers and examine them. Choose one that will become a core - the heart of a new, larger crystal. Look for the ones that meet the following requirements; the criteria are described in descending order of importance:
- Choose a unique crystal that has not come into contact with others;
- It must have flat, uniform surfaces with straight edges;
- It must be as big as at least one bean;
- Find different crystals with these characteristics and transfer them to separate jars as described below; they often melt without developing, so it's worth having more than one.
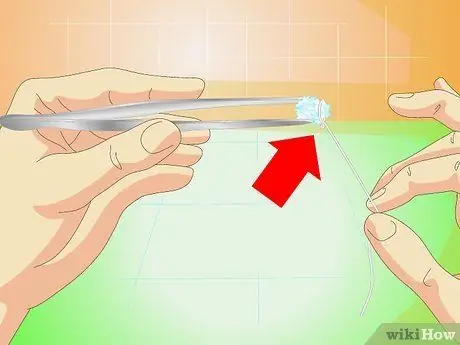
Step 3. Attach a fishing line or smooth metal wire
Use super glue and attach it to one side of the crystal or simply tie the line around the crystal itself.
Do not use coarse thread or twine; you need a smooth surface, otherwise new crystals will grow on the wire instead of the core

Step 4. Prepare a new solution
Take distilled water and the same type of salt; this time it brings the temperature of the liquid slightly beyond that of the environment. Your goal is to get a perfectly saturated solution. If it were unsaturated, it would melt the core; if instead it were supersaturated, the core would be covered with salt grains leading to the formation of crystal clusters.
There are several quick methods to solve the problem, but they are quite complex and require some mastery of chemistry

Step 5. Place the crystal and solution in a clean container
Wash a jar and rinse it with distilled water; pour the new solution into this container and hang the crystal core in the center. Store it respecting the following criteria:
- Place the jar in a cool, dark place, such as in a low kitchen cabinet.
- Place it on top of a piece of Styrofoam or other similar material that absorbs vibrations.
- Cover it with a coffee filter, a sheet of paper or a thin cloth to prevent the solution from being contaminated by dust; do not use an airtight cap.

Step 6. Check the crystal regularly
This time it grows slower, as the water has to evaporate a bit before the salt molecules are forced to adhere to the core. If everything works as it should, the crystal should keep the same shape as it develops. You can take it out of the solution whenever you want, but it is likely to continue growing for several weeks.
- Every two weeks or so, pour the solution through a coffee filter to remove impurities.
- This is a complex procedure. Even experienced crystal "cultivators" sometimes find themselves with a loose core or a shapeless cluster. If you can get the perfect core, you should first test with a less valuable one to see if the solution concentration is correct.
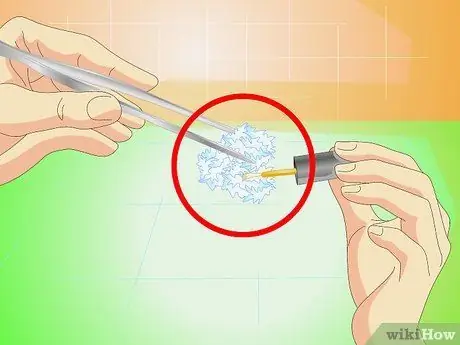
Step 7. Protect the final crystal with nail polish
Once it has reached the desired size, remove it from the liquid and dry it. Apply a coat of clear nail polish to all surfaces to prevent them from deteriorating over time.
Method 3 of 3: Variants
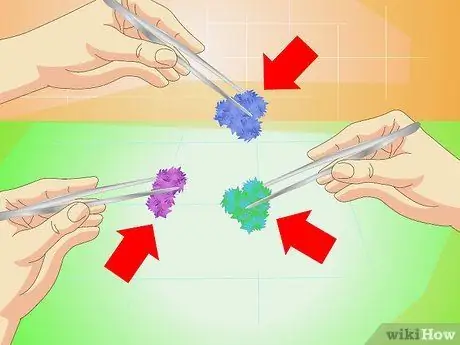
Step 1. Try different substances
There are many substances that crystallize thanks to the techniques described above; some are available at laboratory supply stores. Here are some tips:
- Borax for white or colored crystals
- The cupric sulphate allows to obtain blue crystals;
- Chromium alum generates purple crystals;
- Cupric acetate produces dark, blue-green crystals;
- Attention: These chemicals can be dangerous by inhalation, ingestion or contact with bare skin. Read the safety instructions on the packaging and do not allow children to use them without adult supervision.

Step 2. Make some snowflakes
Tie several pipe cleaners or rough metal wires giving them the shape of a star; soak them in the salt solution and observe the crystals that coat the "star", turning into sparkling snowflakes.
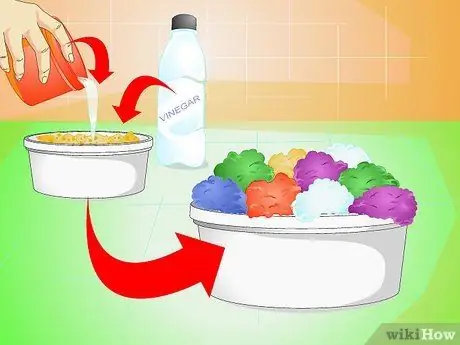
Step 3. Create a crystal garden
Instead of making a single piece, why not make a larger quantity? Prepare the salt solution and pour it over the sponges or pieces of charcoal that you have placed at the bottom of the container; add some vinegar and watch the crystals develop overnight.
- Pour in enough water to completely soak the sponges without submerging them.
- To get crystals of different colors, add a drop of food coloring to each sponge.






