All chemists, biologists, environmentalists and laboratory technicians use pH to measure the acidity or alkalinity of a solution; the pH meter, or pH meter, is very useful and represents the most accurate instrument to measure this value. There are many simple steps, ranging from the preparation of the materials to the methodical calibration of the instrument and its use, which guarantee to obtain measurements with the highest possible precision; you can also measure the pH of the water using specific techniques.
Steps
Part 1 of 3: Preparing to Calibrate the Instrument

Step 1. Turn on the pH meter
Before you start calibrating and using it, you need to turn it on and wait for it to warm up; it usually takes thirty minutes, but consult the manual for precise references.

Step 2. Clean the electrode
Remove it from the package and rinse it with distilled water over an empty bacher that can be used for waste; once rinsed, dry it by dabbing it with a Kimwipe or Shurwipe cloth.
- Remember to rinse the electrode in a different beaker than the one you use to calibrate it.
- Do not rub the probe, as it is covered with a sensitive membrane.
- If you find that it is very dirty, read the manual of the instrument for the recommended cleaning methods.

Step 3. Prepare the buffer solutions
Typically, you need more than one to calibrate the pH meter. The first must be neutral with a pH of 7, the second must have a level similar to the expected level of the sample, be it 4 or 9, 21. Buffer solutions with a high pH (9, 21) are suitable for calibrate the instrument for a basic sample, while those with a low pH (4) are used to prepare the instrument for measuring an acidic sample. When you have chosen them, wait for them to reach the same temperature, as the pH value varies with the temperature. Pour the solutions into individual beakers for calibration.
- Contact your pH meter manufacturer, educational or professional institution to get buffer solutions.
- These should be left in the beakers for no more than two hours.
- Do not pour the solution already used into the original container.
Part 2 of 3: Calibrate the pH meter

Step 1. Put the electrode in the pH 7 buffer solution and take a measurement
Press the activation or calibration button to detect the pH of the solution once the probe is inserted.
Wait for the pH to stabilize before setting it on the meter, leaving the electrode submerged for about a minute or two

Step 2. Set the pH value
When you manage to have a stable and constant reading, set the pH meter to the value of the buffer solution, by pressing the activation or calibration button for a second time; wait for the measured value to stabilize, it allows to obtain a calibration and precise readings.
Although it is not necessary, if you mix the solution before measuring its pH, remember to mix it with all the others, including the sample

Step 3. Rinse the electrode with distilled water
Rinse it and pat it dry with a lint-free cloth, such as Kimwipe or Shurwipe, before dipping it in another buffer solution.

Step 4. Place the electrode in the pH 4 buffer solution and perform a reading
Press the trigger button to measure the pH of the liquid once you have immersed the electrode.
If you are not using the pH 4 solution for calibration, use the pH 9, 21 solution

Step 5. Set the pH value for a second time
When you get a stable and constant reading, set the value by pressing the trigger once more.
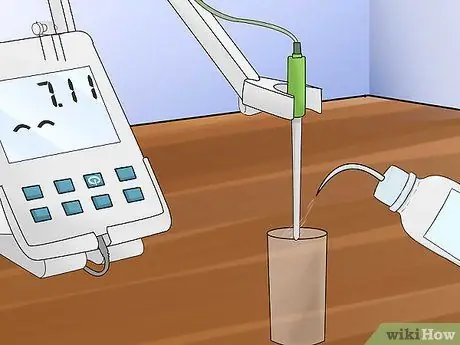
Step 6. Rinse the electrode
Use distilled water for this operation; dry the probe with a lint-free cloth, such as Kimwipe or Shurwipe, before dipping it in another liquid.
Part 3 of 3: Using the pH meter

Step 1. Put the electrode in the sample solution and take the measurement
When the probe is in the liquid, press the activation button and wait about 1-2 minutes without removing it.

Step 2. Set the pH level
When the reading has stabilized, press the activation key; the value you get is the pH of the sample.

Step 3. Clean the electrode after each use
Rinse it with distilled water and pat it dry with a lint-free cloth; once dry and clean, you can store the pH meter.
Consult the user manual to best preserve the specific instrument in your possession
Advice
- If you are unsure about the procedure, ask questions. Ask your lab supervisor or consult the home kit manual.
- Each pH meter is slightly different; check the manual before calibrating and using the instrument in your possession.






